ABOUT
The Pain Research Group is devoted to the understanding of chronic pain through a multidisciplinary approach, which departs from the plastic changes taking place at the nociceptive system when chronic pain develops to the study of specific human pain conditions and their social and economic impact.
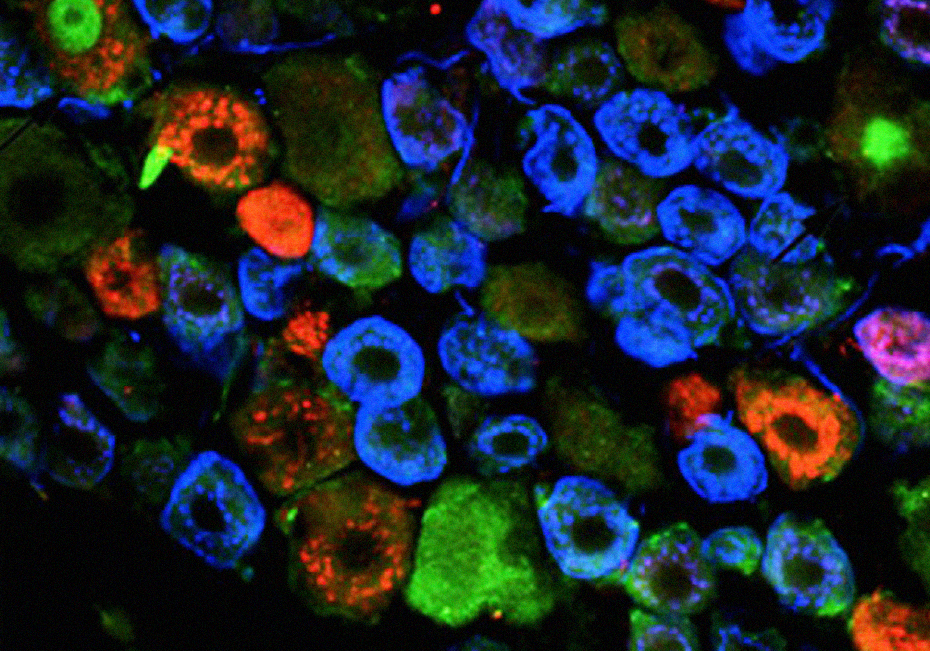
Studies carried on primary afferent nociceptive neurons look for molecules involved in neuronal damage and repair and the mechanisms underlying the neuronal/glia interplay. In parallel, we look for genes involved in the development, differentiation and synaptic connectivity of peripheral and spinal cord nociceptive neurons and their deregulation in chronic pain conditions.
Research on the supraspinal pain control system combines circuit tracing, measuring of neurotransmitter release and gene expression manipulation to address the role of descending pain facilitation and its interaction with the inhibitory system in the establishment of chronic pain. Under focus are the aminergic system and its interface with the opioid and GABA systems.
The affective-cognitive impairment induced by chronic pain and the underlying circuitry changes are studied by correlating in vivo electrophysiology with animal behavior on one side and neurochemical characterization of circuits involved, on the other. Parallel studies using psychophysical and neuroimaging approaches investigate how pain processing is affected in a number of clinical pain conditions disturbing emotional and cognitive processing.
Finally, epidemiological studies are carried on to determine the social and economic impact of pain in Portugal.
RESEARCH
Chronic pain affects 37% of the Portuguese population and leads to total annual costs of € 4600 million.
In chronic pain, nociceptors exhibit increased expression of injury and regeneration markers, while at the spinal cord, potassium chloride co-transporter is underexpressed, with marked glial activation.
Prrxl1 is essential for the development of nociceptor-spinal citcuit by commanding the differentiation of spinal excitatory neurons and guiding nociceptors to their spinal targets. Tlx3 interacts with Prrxl1 promotor to induce diferential Prrxl1 hyperphosphorilation in DRG and spinal neurons.
Noradrenalin and serotonin decrease in pain inhibitory brain areas, but noradrenalin still drives pain trhough activation of the pro-nociceptive center, DRt. Facilitation from the DRt is endogenously reduced during chronic pain through an increase of spinal GABAergic inhibition. Gene transfer-mediated reduction of noradrenalin release at the DRt or spinal administration of GABABR agonists result in marked analgesia.
Pain-induced changes in spatial memory and in strategic planning and risk assessment correlate with, respectively, disrupted hippocampal encoding and decreased prefrontal activity.