Cell Biology of Bacterial Infections
The plasma membrane serves as a vital cellular barrier, constantly facing assaults from external factors. Plasma membrane damage frequently occurs under physiological conditions, especially in tissues subjected to mechanical or biochemical stress, such as the gut, muscles, and skin. To cope with damage, cells possess intrinsic self-repair capabilities through mechanisms that remain poorly elucidated. However, it appears that these mechanisms are conserved across various sources of damage.
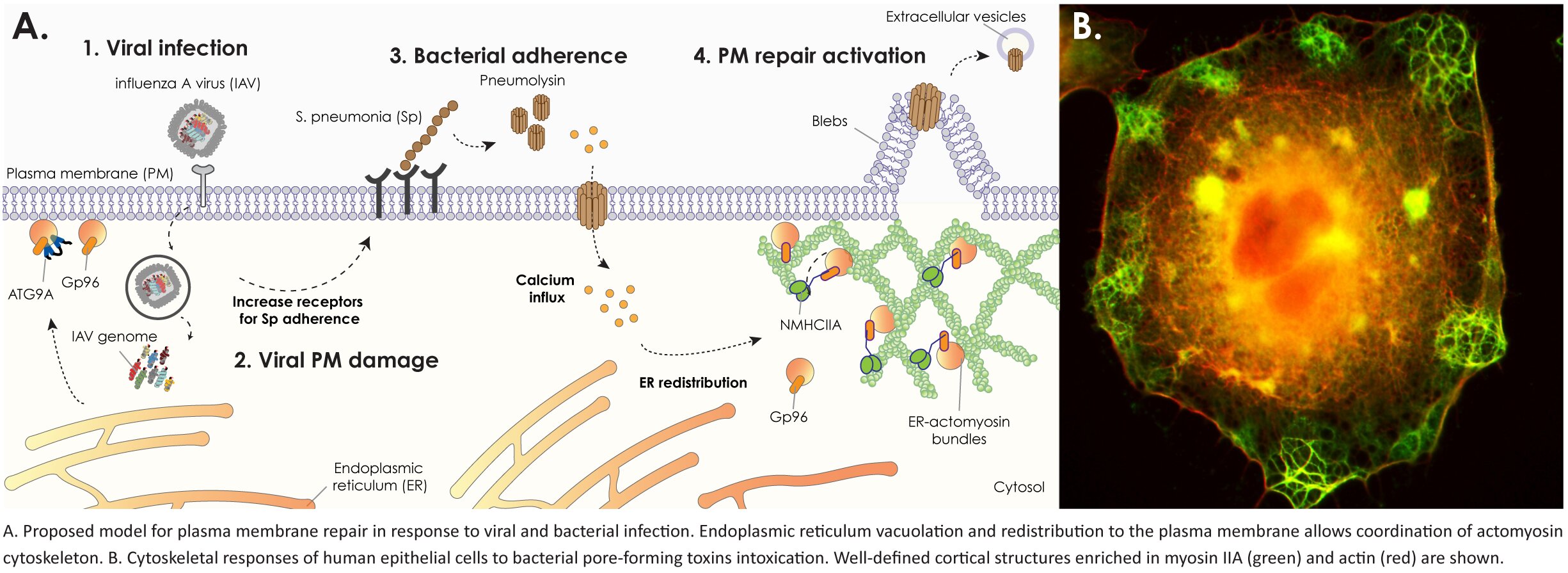
During infections, pathogens strategically target the integrity of the host plasma membrane to disrupt epithelial barriers, manipulate the immune response, and facilitate their dissemination. Several highly pathogenic human bacteria secrete pore-forming toxins, which serve as major virulence factors by inserting in the host cell’s plasma membrane, creating transmembrane pores and thus enabling the pathogens to manipulate their host environment and establish infection. Our research delves into the molecular intricacies of plasma membrane repair mechanisms activated in response to infectious damage.
RESEARCH
We are deeply immersed in unraveling the intricate intracellular processes governing plasma membrane rearrangements and actomyosin cytoskeleton remodeling, which are pivotal events for membrane resealing and thus constitute a critical aspect of cell survival and defense against infections. By elucidating these mechanisms, we aim to enrich our comprehension of how host cells endure infectious damage, thereby laying the groundwork for innovative therapeutic strategies to combat infections and promote tissue repair.
The disruption of plasma membrane integrity triggers uncontrolled exchanges between the intracellular and extracellular environments, precipitating the loss of cellular homeostasis. Notably, our research has revealed that the massive influx of calcium through the toxin-based pores induces endoplasmic reticulum (ER) disruption and prompts the recruitment of ER-derived vacuoles to the cell cortex. This process occurs concurrently with profound remodeling of the cortical actomyosin cytoskeleton and extensive plasma membrane blebbing. Importantly, we have established a correlation between plasma membrane blebbing, actomyosin cytoskeleton remodeling, and the resolution of damage, demonstrating their regulation by ER proteins.
Based on available data, we postulate that the recruitment of ER-derived vacuoles to the cell cortex serves a dual purpose: firstly, as a source of new membrane for damage repair, and secondly, to polarize essential intracellular machinery to the site of injury. To further investigate our hypothesis, we employ a multifaceted approach encompassing proximity-labeling proteomics, live-cell imaging, and advanced high-resolution and combinatory electron microscopy techniques.
To induce damage in host cell lines and deeply investigate common repair mechanisms, we employ a variety of damaging agents, including:
- Purified recombinant pore-forming toxins, such as PLY (Pneumolysin), LLO (Listeriolysin O), and PFO (Perfringolysin O);
- Bacterial pathogens known to produce pore-forming toxins, including Streptococcus pneumoniae, Listeria monocytogenes, and Clostridium perfringens;
- Influenza A virus, which causes significant damage to epithelial lung cells.
In our laboratory, we employ a diverse range of cellular models for our research. These include commercially available epithelial cell lines, genetically modified cell lines, 3D cell models cultured at the air-liquid interface, and stem cell-derived 3D cell models. To evaluate infection outcomes, we commonly utilize zebrafish or C. elegans infection models.
We are currently addressing several key issues, including:
- Characterizing novel repair machineries, with a specific emphasis on calcium-responsive proteins;
- Resolving the cellular ultrastructure in the vicinity of the pore and the plasma membrane blebbing, to uncover new cellular compartments involved in the repair process;
- Elucidating the molecular intricacies of the crosstalk between the endoplasmic reticulum (ER) and cytoskeletal components;
- Investigating the spatiotemporal regulation of repair events.
Collaborators
- J. Leong, Tufts University, Boston-USA
- E. Bou Ghanem, University at Buffalo, Buffalo-USA
- A. Lacy-Hulbert, Benaroya Research Institute, Seattle-USA
- S. Mostowy, Imperial College, London-UK
- J. Sellers, NIH-NHLBI, Bethesda-USA
- F. Impens, VIB-UGhent, Gent-Belgium
- D. Cabanes, i3S, Porto-Portugal
- A. X. Carvalho, i3S, Porto-Portugal
Team
Selected Publications
Cells Responding to Closely Related Cholesterol-Dependent Cytolysins Release Extracellular Vesicles with a Common Proteomic Content Including Membrane Repair Proteins. Toxins15(1):, 2023. [Journal: Article] [CI: 3] [IF: 4,2 (*)]
DOI: 10.3390/toxins15010004 SCOPUS: 85146784684
Brito C., Pereira J.M., Mesquita F.S., Cabanes D., Sousa S.
Src-Dependent NM2A Tyrosine Phosphorylation Regulates Actomyosin Remodeling. Cells12(14):, 2023. [Journal: Article] [CI: 1] [IF: 6,0 (*)]
DOI: 10.3390/cells12141871 SCOPUS: 85166001196
Pereira J.M., Xu S., Leong J.M., Sousa S.
The Yin and Yang of Pneumolysin During Pneumococcal Infection. Frontiers in Immunology13:, 2022. [Journal: Review] [CI: 25] [IF: 7,3]
DOI: 10.3389/fimmu.2022.878244 SCOPUS: 85129775459
Costa A.C., Carvalho F., Cabanes D., Sousa S.
Stathmin recruits tubulin to Listeria monocytogenes-induced actin comets and promotes bacterial dissemination. Cellular and Molecular Life Sciences76(5):961-975, 2019. [Journal: Article] [CI: 6] [IF: 6,5]
DOI: 10.1007/s00018-018-2977-7 SCOPUS: 85057542589
Brito C., Cabanes D., Sarmento Mesquita F., Sousa S.
Mechanisms protecting host cells against bacterial pore-forming toxins. Cellular and Molecular Life Sciences76(7):1319-1339, 2019. [Journal: Review] [CI: 103] [IF: 6,5]
DOI: 10.1007/s00018-018-2992-8 SCOPUS: 85059153781
Pinheiro J., Lisboa J., Pombinho R., Carvalho F., Carreaux A., Brito C., Pöntinen A., Korkeala H., Dos Santos N.M.S., Morais-Cabral J.H., Sousa S., Cabanes D.
MouR controls the expression of the Listeria monocytogenes Agr system and mediates virulence. Nucleic Acids Research46(18):9338-9352, 2018. [Journal: Article] [CI: 32] [IF: 11,1]
DOI: 10.1093/nar/gky624 SCOPUS: 85054896319
Mesquita F.S., Brito C., Mazon Moya M.J., Pinheiro J.C., Mostowy S., Cabanes D., Sousa S.
Endoplasmic reticulum chaperone Gp96 controls actomyosin dynamics and protects against pore-forming toxins. EMBO Reports18(2):303-318, 2017. [Journal: Article] [CI: 23] [IF: 8,7]
DOI: 10.15252/embr.201642833 SCOPUS: 85007487818
Almeida M.T., Mesquita F.S., Cruz R., Osório H., Custódio R., Brito C., Vingadassalom D., Martins M., Leong J.M., Holden D.W., Cabanes D., Sousa S.
Src-dependent tyrosine phosphorylation of non-muscle myosin heavy chain-IIA restricts Listeria monocytogenes cellular infection. Journal of Biological Chemistry290(13):8383-8395, 2015. [Journal: Article] [CI: 19] [IF: 4,3]
DOI: 10.1074/jbc.M114.591313 SCOPUS: 84925760833
Leitão E., Costa A.C., Brito C., Costa L., Pombinho R., Cabanes D., Sousa S.
Listeria monocytogenes induces host DNA damage and delays the host cell cycle to promote infection. Cell Cycle13(6):928-940, 2014. [Journal: Article] [CI: 31] [IF: 4,6]
DOI: 10.4161/cc.27780 SCOPUS: 84897994129
Martins M., Custod́io R., Camejo A., Almeida M.T., Cabanes D., Sousa S.
Listeria monocytogenes triggers the cell surface expression of Gp96 protein and interacts with its N terminus to support cellular infection. Journal of Biological Chemistry287(51):43083-43093, 2012. [Journal: Article] [CI: 28] [IF: 4,7]
DOI: 10.1074/jbc.M112.422568 SCOPUS: 84871163192


