Hematopoiesis and Microenvironments
ABOUT
We aim to discover ecological interactions in normal and malignant hematopoiesis by studying:
- Niche determinants of clonal heterogeneity, expansion and relapse in leukemia
- Spatial distribution, competition and cooperation between leukemia clones
- Remodeling and targeting of vascular microenvironments in leukemia and lymphoma
- Physiologic and long-range regulatory systems of hematopoiesis and leukemia
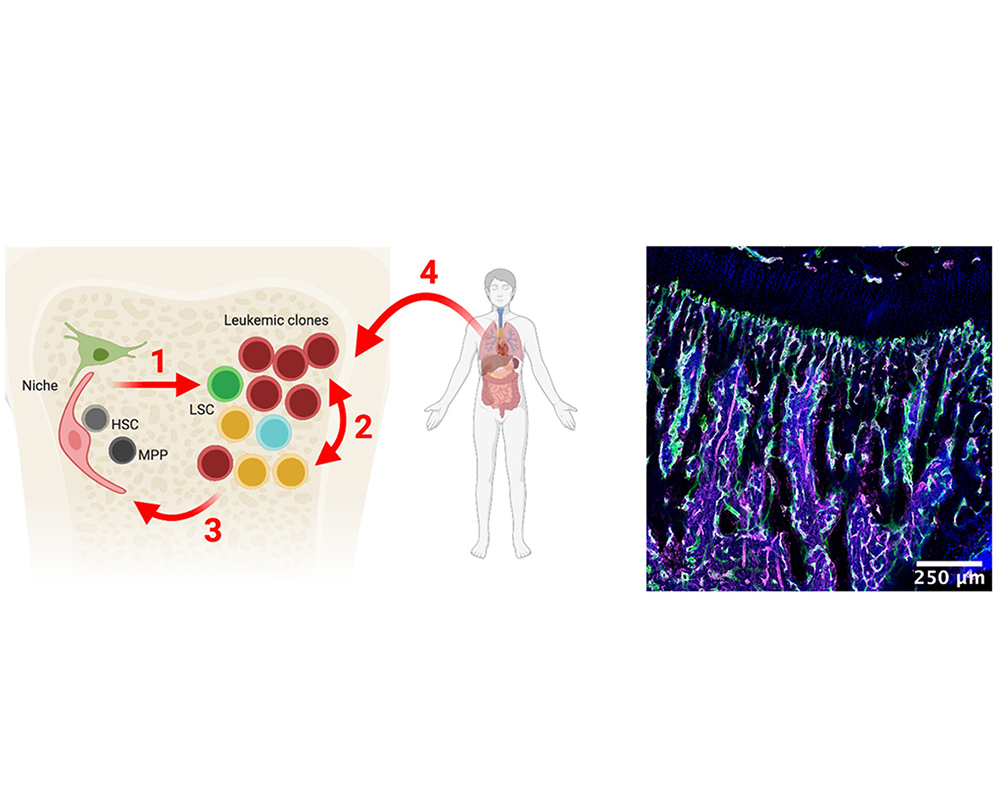
RESEARCH
Our lab aims to study the cell-extrinsic regulation of normal and malignant hematopoietic cells at the local and systemic levels, specifically focusing on acute myeloid leukemia (AML) but also on of T-cell acute lymphoblastic leukemia (T-ALL) and B-cell lymphoma.
Landmark studies have deciphered genetic alterations that occur in blood cancers and how this drives their complex clonal structure. Yet, we lack fundamental knowledge of the cell-to-cell interactions occurring between normal and malignant hematopoietic cells and their neighbors in the bone marrow (BM) niche. Specifically, the interaction between leukemic stem cells (LSCs) and niches and the dynamics of inter-clonal competition and cooperation in leukemia remain underexplored. We have previously used intravital microscopy of the mouse BM to record the in vivo behavior of T-cell acute lymphoblastic leukemia (T-ALL) and acute myeloid leukemia (AML) at the single-cell level and to demonstrate how local niches are remodeled in leukemia. More recently, we have shown how iron metabolism is dysregulated in AML, both at the local and systemic levels. Our lab uses pre-clinical mouse models of disease, mouse mutants that enable in vivo mechanistic studies, advanced BM imaging, primary patient samples, cell lines and curated clinical data. We have a collaborative environment and benefit from a close interaction with the clinic, particularly IPO-Porto, as part of the Porto Comprehensive Cancer Center.
If you are interested in discussing our work or applying for a position, please contact Delfim Duarte (delfimd@med.up.pt).
Team
Selected Publications
Nanoparticle-encapsulated retinoic acid for the modulation of bone marrow hematopoietic stem cell niche. Bioactive Materials34:311-325, 2024. [Journal: Article] [CI: 8] [IF: 20.3]
DOI: 10.1016/j.bioactmat.2023.12.017 SCOPUS: 85181839732
Duarte T.L., Lopes M., Oliveira M., Santos A.G., Vasco C., Reis J.P., Antunes A.R., Gonçalves A., Chacim S., Oliveira C., Porto B., Teles M.J., Moreira A.C., Silva A.M.N., Schwessinger R., Drakesmith H., Henrique R., Porto G., Duarte D.
Iron overload induces dysplastic erythropoiesis and features of myelodysplasia in Nrf2-deficient mice. Leukemia:, 2023. [Journal: Article] [CI: 3] [IF: 12.8]
DOI: 10.1038/s41375-023-02067-9 SCOPUS: 85174512949
Mosteo L., Reis J., Rocha L., Lopes M., Duarte D.
Flow Cytometry Analysis of Murine Bone Marrow Hematopoietic Stem and Progenitor Cells and Stromal Niche Cells. Journal of Visualized Experiments2022(187):, 2022. [Journal: Article] [CI: 1] [IF: 1,2]
DOI: 10.3791/64248 SCOPUS: 85139125813
Pirillo C., Birch F., Tissot F.S., Anton S.G., Haltalli M., Tini V., Kong I., Piot C., Partridge B., Pospori C., Keeshan K., Santamaria S., Hawkins E., Falini B., Marra A., Duarte D., Lee C.F., Roberts E., Celso C.L.
Metalloproteinase inhibition reduces AML growth, prevents stem cell loss, and improves chemotherapy effectiveness. Blood Advances6(10):3126-3141, 2022. [Journal: Article] [CI: 16] [IF: 7,5]
DOI: 10.1182/bloodadvances.2021004321 SCOPUS: 85130609185
Lopes M., Duarte T.L., Teles M.J., Mosteo L., Chacim S., Aguiar E., Pereira-Reis J., Oliveira M., Silva A.M.N., Goncalves N., Martins G., Kong I.Y., Zethoven M., Vervoort S., Martins S., Quintela M., Hawkins E.D., Trigo F., Guimaraes J.T., Mariz J.M., Porto G., Duarte D.
Loss of erythroblasts in acute myeloid leukemia causes iron redistribution with clinical implications. Blood Advances5(16):3102-3112, 2021. [Journal: Article] [CI: 12] [IF: 7,6]
DOI: 10.1182/bloodadvances.2021004373 SCOPUS: 85113994709
Sinha S., Pereira-Reis J., Guerra A., Rivella S., Duarte D.
The Role of Iron in Benign and Malignant Hematopoiesis. Antioxidants and Redox Signaling35(6):415-432, 2021. [Journal: Review] [CI: 23] [IF: 7,5]
DOI: 10.1089/ars.2020.8155 SCOPUS: 85105672354
Mosteo L., Storer J., Batta K., Searle E.J., Duarte D., Wiseman D.H.
The Dynamic Interface Between the Bone Marrow Vascular Niche and Hematopoietic Stem Cells in Myeloid Malignancy. Frontiers in Cell and Developmental Biology9:, 2021. [Journal: Review] [CI: 26] [IF: 6,1]
DOI: 10.3389/fcell.2021.635189 SCOPUS: 85103027137
Duarte D., Amarteifio S., Ang H., Kong I.Y., Ruivo N., Pruessner G., Hawkins E.D., Lo Celso C.
Defining the in vivo characteristics of acute myeloid leukemia cells behavior by intravital imaging. Immunology and Cell Biology97(2):229-235, 2019. [Journal: Article] [CI: 25] [IF: 3,7]
DOI: 10.1111/imcb.12216 SCOPUS: 85058390194
Duarte D., Hawkins E.D., Akinduro O., Ang H., De Filippo K., Kong I.Y., Haltalli M., Ruivo N., Straszkowski L., Vervoort S.J., McLean C., Weber T.S., Khorshed R., Pirillo C., Wei A., Ramasamy S.K., Kusumbe A.P., Duffy K., Adams R.H., Purton L.E., Carlin L.M., Lo Celso C.
Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell22(1):64-77.e6, 2018. [Journal: Article] [CI: 244] [IF: 21,5]
DOI: 10.1016/j.stem.2017.11.006 SCOPUS: 85038843065
Hawkins E.D., Duarte D., Akinduro O., Khorshed R.A., Passaro D., Nowicka M., Straszkowski L., Scott M.K., Rothery S., Ruivo N., Foster K., Waibel M., Johnstone R.W., Harrison S.J., Westerman D.A., Quach H., Gribben J., Robinson M.D., Purton L.E., Bonnet D., Lo Celso C.
T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature538(7626):518-522, 2016. [Journal: Article] [CI: 169] [IF: 40,1]
DOI: 10.1038/nature19801 SCOPUS: 84994005268


