Expression Regulation in Cancer
ABOUT
Our general goal is to disclose germline and somatic mechanisms increasing susceptibility to gastric cancer, and conferring advantageous features to tumour cells. This work is expected to provide novel diagnostic tools and targetable cancer cell fragilities for therapeutic intervention. The team is also interested in understanding if application of cancer prevention measures in carriers of tumour-predisposing germline variants is cost-effective.
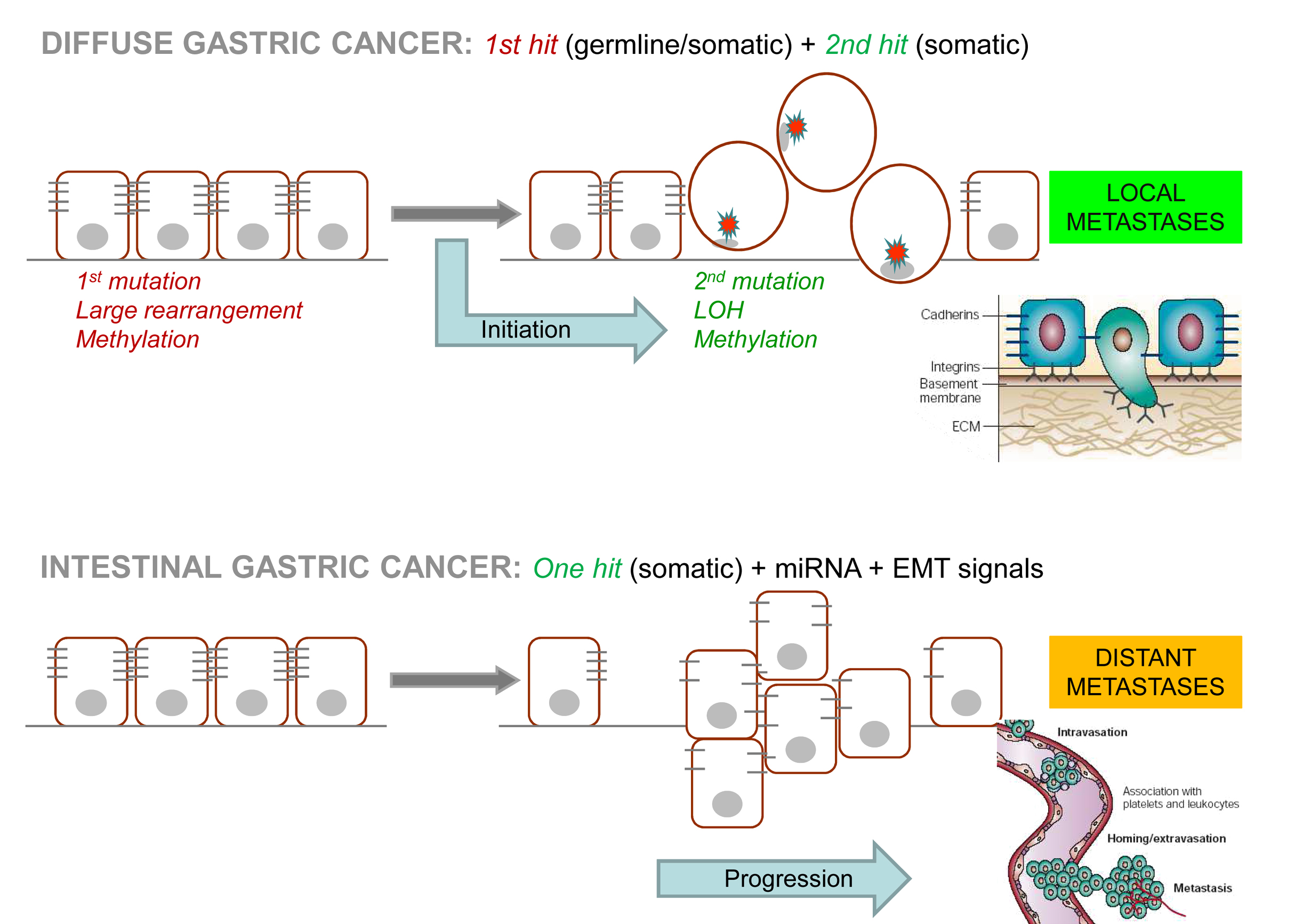
We pioneered the discovery of CDH1 germline deletions as a cause of Hereditary Diffuse Gastric Cancer (HDGC), and found that deletions in regulatory sequences, downstream of the CDH1 locus, cause earlier disease onset and increase disease penetrance. We recently demonstrated that carriers of CDH1-truncating variants have increased risk to develop lobular breast cancer and diffuse gastric cancer, compared to carriers of CDH1-missense variant. A large statistical analysis allowed us to propose new clinical criteria for CDH1 germline testing, which we demonstrated to improve testing sensitivity while retaining high specificity to identify patients and families at higher cancer risk. Our group has also designed, fabricated and tested the first multi-layered Stomach-on-a-chip with mechanical actuation mimicking gastric peristalsis. This is a particular relevant asset to explore molecular mechanisms involved in gastric cancer initiation and progression.
Our long-term goal is to improve prevention of gastric cancer onset, as well as clinical management and cancer treatment in patients diagnosed with hereditary gastric cancer.
RESEARCH
Our research objectives are:
- Identifying causal germline defects underlying hereditary gastric cancer development and genotype-phenotype correlations, using real patient data, statistical tools and functional genomics (integration of large clinical datasets, omics technologies, organoids and animal models such as zebrafish, drosophila, mouse);
- Identifying of lifetime cancer risks in carriers of CDH1 and CTNNA1 predisposing variants, and genetic modifiers of disease penetrance and expressivity, using large clinical datasets and statistical tools, patient-derived induced Pluripotent Stem Cells (iPSCs) and organoids;
- Designing and fabricating organ-on-a-chip platforms to explore initial steps of gastric cancer development, metastatic spread and therapeutic approaches.
- Obtaining the cost-benefit of risk-reduction interventions in Rare Tumours Risk Syndromes (RTRS), to ultimately settle cost-effective RTRS patient-focused care at the European level.
Our group is multidisciplinary and composed of basic and clinical scientists. We have a strong scientific background in genetics and oncobiology, supported by technical expertise in molecular and cellular biology, Next-Generation Sequencing and Bioinformatics, organ-on-a-chip, iPSCs, organoids and animal models. Through national and international collaborations, the group has access to large clinical and molecular datasets from hereditary and sporadic cancer patients’ cohorts, as well as to high level discussions with expert scientists and clinicians.
Expression Regulation in Cancer group members have in general international exposure by participation in international consortia and scientific societies, as well as training in international laboratories and networking through mobility and exchange programs. To achieve this, the group actively engages in networking activities promoting collaborations with researchers and hospitals worldwide. Examples of these networks are the European Reference Network on Tumours Risk Syndromes (ERN-GENTURIS), The SolveRD consortium; the Preventable consortium; TrainEV Marie Sklodowska-Curie Action; the IGCLC – International Gastric Cancer Linkage Consortium, and the CDH1 Variant Curation Expert Panel.
Team
Selected Publications
Genotype-first approach to identify associations between CDH1 germline variants and cancer phenotypes: a multicentre study by the European Reference Network on Genetic Tumour Risk Syndromes. The Lancet Oncology24(1):91-106, 2023. [Journal: Article] [CI: 19] [IF: 41.6]
DOI: 10.1016/S1470-2045(22)00643-X SCOPUS: 85145669093
Blair V.R., McLeod M., Carneiro F., Coit D.G., D'Addario J.L., van Dieren J.M., Harris K.L., Hoogerbrugge N., Oliveira C., van der Post R.S., Arnold J., Benusiglio P.R., Bisseling T.M., Boussioutas A., Cats A., Charlton A., Schreiber K.E.C., Davis J.L., Pietro M.d., Fitzgerald R.C., Ford J.M., Gamet K., Gullo I., Hardwick R.H., Huntsman D.G., Kaurah P., Kupfer S.S., Latchford A., Mansfield P.F., Nakajima T., Parry S., Rossaak J., Sugimura H., Svrcek M., Tischkowitz M., Ushijima T., Yamada H., Yang H.K., Claydon A., Figueiredo J., Paringatai K., Seruca R., Bougen-Zhukov N., Brew T., Busija S., Carneiro P., DeGregorio L., Fisher H., Gardner E., Godwin T.D., Holm K.N., Humar B., Lintott C.J., Monroe E.C., Muller M.D., Norero E., Nouri Y., Paredes J., Sanches J.M., Schulpen E., Ribeiro A.S., Sporle A., Whitworth J., Zhang L., Reeve A.E., Guilford P.
Hereditary diffuse gastric cancer: updated clinical practice guidelines. The Lancet Oncology21(8):e386-e397, 2020. [Journal: Review] [CI: 275] [IF: 41,3]
DOI: 10.1016/S1470-2045(20)30219-9 SCOPUS: 85088930288
Ferreira D.A., Conde J.P., Rothbauer M., Ertl P., Granja P.L., Oliveira C.
Bioinspired human stomach-on-a-chip with in vivo like function and architecture. Lab on a Chip23(3):495-510, 2023. [Journal: Article] [CI: 12] [IF: 6.1]
DOI: 10.1039/d2lc01132h SCOPUS: 85146176758
Ferreira D.A., Rothbauer M., Conde J.P., Ertl P., Oliveira C., Granja P.L.
A Fast Alternative to Soft Lithography for the Fabrication of Organ-on-a-Chip Elastomeric-Based Devices and Microactuators. Advanced Science8(8):, 2021. [Journal: Article] [CI: 26] [IF: 17,5]
DOI: 10.1002/advs.202003273 SCOPUS: 85100571451
São José C., Garcia-Pelaez J., Ferreira M., Arrieta O., André A., Martins N., Solís S., Martínez-Benítez B., Ordóñez-Sánchez M.L., Rodríguez-Torres M., Sommer A.K., te Paske I.B.A.W., Caldas C., Tischkowitz M., Tusié M.T., Aretz S., Capella G., Castedo S., Evans G., Fernandes S., Garrido L., Holinski-Feder E., Huntsman D., Jahn A., Kets C.M., Laner A., Ligtenberg M., Meinhardt A., Mensenkamp A., Oliveira C., Peters S., Quintana I., Schröck E., Spier I., Spruijt L., Steinke-Lange V., Paske I.t., Valle L., van der Post R., van Herwaarden Y., van Zelst-Stams W., William D., Hoogerbrugge N., Demidov G., de Voer R.M., Laurie S., Oliveira C.
Combined loss of CDH1 and downstream regulatory sequences drive early-onset diffuse gastric cancer and increase penetrance of hereditary diffuse gastric cancer. Gastric Cancer26(5):653-666, 2023. [Journal: Article] [CI: 5] [IF: 6]
DOI: 10.1007/s10120-023-01395-0 SCOPUS: 85160585792
Garcia-Pelaez J., Barbosa-Matos R., Gullo I., Carneiro F., Oliveira C.
Histological and mutational profile of diffuse gastric cancer: current knowledge and future challenges. Molecular Oncology15(11):2841-2867, 2021. [Journal: Review] [CI: 28] [IF: 7,4]
DOI: 10.1002/1878-0261.12948 SCOPUS: 85117071465
Barbosa-Matos R., Silva R.L., Garrido L., Aguiar A.C., Garcia-Pelaez J., André A., Seixas S., Sousa S.P., Ferro L., Vilarinho L., Gullo I., Devezas V., Oliveira R., Fernandes S., Costa S.C., Magalhães A., Baptista M., Carneiro F., Pinheiro H., Castedo S., Oliveira C.
The cdh1 c.1901c>t variant: A founder variant in the portuguese population with severe impact in mrna splicing. Cancers13(17):, 2021. [Journal: Article] [CI: 7] [IF: 6,6]
DOI: 10.3390/cancers13174464 SCOPUS: 85114263066
Lobo S., Benusiglio P.R., Coulet F., Boussemart L., Golmard L., Spier I., Hüneburg R., Aretz S., Colas C., Oliveira C.
Cancer predisposition and germline CTNNA1 variants. European Journal of Medical Genetics64(10):, 2021. [Journal: Article] [CI: 28] [IF: 2,5]
DOI: 10.1016/j.ejmg.2021.104316 SCOPUS: 85113340427
Pereira C., Park J.H., Campelos S., Gullo I., Lemos C., Solorzano L., Martins D., Gonçalves G., Leitão D., Lee H.J., Kong S.H., André A., Borges C., Almeida D., Wälbhy C., Almeida R., Kim W.H., Carneiro F., Yang H.K., Almeida G.M., Oliveira C.
Comparison of East-Asia and West-Europe cohorts explains disparities in survival outcomes and highlights predictive biomarkers of early gastric cancer aggressiveness. International Journal of Cancer150(5):868-880, 2022. [Journal: Article] [CI: 8] [IF: 6,4]
DOI: 10.1002/ijc.33872 SCOPUS: 85121371701
Rocha S., Carvalho J., Oliveira P., Voglstaetter M., Schvartz D., Thomsen A.R., Walter N., Khanduri R., Sanchez J.C., Keller A., Oliveira C., Nazarenko I.
3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles. Advanced Science6(4):, 2019. [Journal: Article] [CI: 103] [IF: 15,8]
DOI: 10.1002/advs.201800948 SCOPUS: 85058843482