Immune Regulation
ABOUT
The immune system has evolved several regulatory mechanisms to achieve maximum protection in the absence of pathology. Pathogens have co-evolved strategies to evade the host immune response. The Immune Regulation group is dedicated to the understanding of the mechanisms explored by hosts and pathogens to modulate the immune response and of the events leading to disease when this regulation fails.
RESEARCH
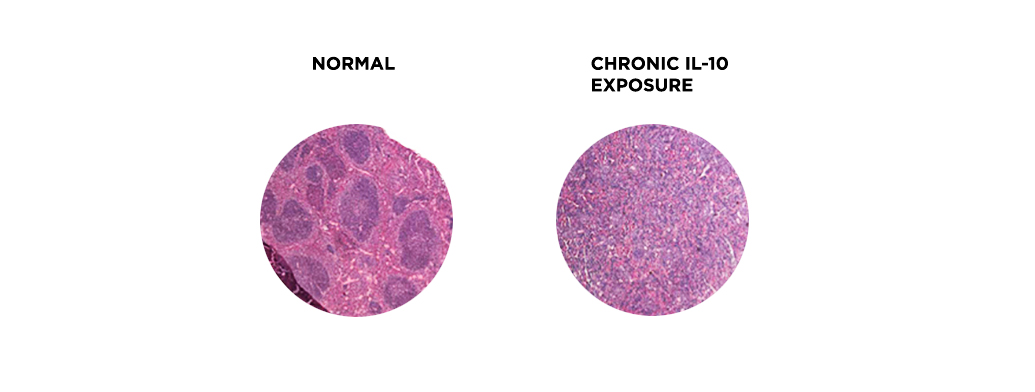
On the host side, we study the anti-inflammatory cytokine IL-10. As this molecule has attracted much attention for potential clinical applications, understanding the molecular mechanisms underlying both IL-10 expression and IL-10 action in immune cells is critical for a better and safer manipulation of the immune system. Our recent data revealed a role for IL-10 in hematopoiesis, by promoting myelopoiesis, and in accelerating aging-related features. We have evidence that IL-10 is accelerating cellular senescence and are pursuing these findings further.
On the pathogen side, we investigate how Mycobacterium tuberculosis, a pathogen that kills over 1.5 million people per year, interacts with the host immune system. We are exploring the physiopathology of tuberculosis in a novel, integrative way, by bringing together the host, the pathogen and the environment diversity. We center our efforts in unveiling the molecular bases implicated in the induction of variable inflammatory responses by M. tuberculosis clinical isolates in different hosts and how these strategies of immune regulation can be explored towards the host benefit. We also investigate how perturbations on the host status (as imposed by comorbidities such as diabetes and cancer) impact on the immune interactions with M. tuberculosis.
Our collaborative network included different groups within i3S, clinicians, and research teams at the Pasteur Institute (Paris), the Francis Crick Institute (London), the Swiss Tropical and Public Health Institute (Basel) and the Biomedicine Institute (Valencia).
Team
Selected Publications
Infection with hypervirulent Mycobacterium tuberculosis triggers emergency myelopoiesis but not trained immunity. Frontiers in Immunology14:, 2023. [Journal: Article] [CI: 9] [IF: 7,3 (*)]
DOI: 10.3389/fimmu.2023.1211404 SCOPUS: 85163978125
Cardoso A., Martins A.C., Maceiras A.R., Liu W., Castro I., Castro A.G., Bandeira A., Di Santo J.P., Cumano A., Li Y., Vieira P., Saraiva M.
Interleukin-10 induces interferon-γ-dependent emergency myelopoiesis. Cell Reports37(4):, 2021. [Journal: Article] [CI: 24] [IF: 10]
DOI: 10.1016/j.celrep.2021.109887 SCOPUS: 85119977049
Sousa J., Cá B., Maceiras A.R., Simões-Costa L., Fonseca K.L., Fernandes A.I., Ramos A., Carvalho T., Barros L., Magalhães C., Chiner-Oms Á., Machado H., Veiga M.I., Singh A., Pereira R., Amorim A., Vieira J., Vieira C.P., Bhatt A., Rodrigues F., Rodrigues P.N.S., Gagneux S., Castro A.G., Guimarães J.T., Bastos H.N., Osório N.S., Comas I., Saraiva M.
Mycobacterium tuberculosis associated with severe tuberculosis evades cytosolic surveillance systems and modulates IL-1β production. Nature Communications11(1):, 2020. [Journal: Article] [CI: 72] [IF: 14,9]
DOI: 10.1038/s41467-020-15832-6 SCOPUS: 85083842157
Fonseca K.L., Maceiras A.R., Matos R., Simoes-Costa L., Sousa J., Cá B., Barros L., Fernandes A.I., Mereiter S., Reis R., Gomes J., Tapia G., Rodríguez-Martínez P., Martín-Céspedes M., Vashakidze S., Gogishvili S., Nikolaishvili K., Appelberg R., Gärtner F., Rodrigues P.N.S., Vilaplana C., Reis C.A., Magalhães A., Saraiva M.
Deficiency in the glycosyltransferase Gcnt1 increases susceptibility to tuberculosis through a mechanism involving neutrophils. Mucosal Immunology13(5):836-848, 2020. [Journal: Article] [CI: 23] [IF: 7,3]
DOI: 10.1038/s41385-020-0277-7 SCOPUS: 85082197621
Moreira-Teixeira L., Stimpson P.J., Stavropoulos E., Hadebe S., Chakravarty P., Ioannou M., Aramburu I.V., Herbert E., Priestnall S.L., Suarez-Bonnet A., Sousa J., Fonseca K.L., Wang Q., Vashakidze S., Rodríguez-Martínez P., Vilaplana C., Saraiva M., Papayannopoulos V., O’Garra A.
Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nature Communications11(1):, 2020. [Journal: Article] [CI: 154] [IF: 14,9]
DOI: 10.1038/s41467-020-19412-6 SCOPUS: 85094939183
Moreira-Teixeira L., Tabone O., Graham C.M., Singhania A., Stavropoulos E., Redford P.S., Chakravarty P., Priestnall S.L., Suarez-Bonnet A., Herbert E., Mayer-Barber K.D., Sher A., Fonseca K.L., Sousa J., Cá B., Verma R., Haldar P., Saraiva M., O’Garra A.
Mouse transcriptome reveals potential signatures of protection and pathogenesis in human tuberculosis. Nature Immunology21(4):464-476, 2020. [Journal: Article] [CI: 88] [IF: 25,6]
DOI: 10.1038/s41590-020-0610-z SCOPUS: 85082258302
Cá B., Fonseca K.L., Sousa J., Maceiras A.R., Machado D., Sanca L., Rabna P., Rodrigues P.N.S., Viveiros M., Saraiva M.
Experimental Evidence for Limited in vivo Virulence of Mycobacterium africanum. Frontiers in Microbiology10:, 2019. [Journal: Article] [CI: 13] [IF: 4,2]
DOI: 10.3389/fmicb.2019.02102 SCOPUS: 85072951370
Moreira-Teixeira L., Sousa J., McNab F.W., Torrado E., Cardoso F., Machado H., Castro F., Cardoso V., Gaifem J., Wu X., Appelberg R., Castro A.G., O'Garra A., Saraiva M.
Type i IFN inhibits alternative macrophage activation during mycobacterium tuberculosis infection and leads to enhanced protection in the absence of IFN-γ signaling. Journal of Immunology197(12):4714-4726, 2016. [Journal: Article] [CI: 82] [IF: 4,9]
DOI: 10.4049/jimmunol.1600584 SCOPUS: 85002328285
Carmona J., Cruz A., Moreira-Teixeira L., Sousa C., Sousa J., Osorio N.S., Saraiva A.L., Svenson S., Kallenius G., Pedrosa J., Rodrigues F., Castro A.G., Saraiva M.
Mycobacterium tuberculosis Strains Are Differentially Recognized by TLRs with an Impact on the Immune Response. PLoS ONE8(6):, 2013. [Journal: Article] [CI: 73] [IF: 3,5]
DOI: 10.1371/journal.pone.0067277 SCOPUS: 84879468975
Cardoso A., Castro A.G., Martins A.C., Carriche G.M., Murigneux V., Castro I., Cumano A., Vieira P., Saraiva M.
The Dynamics Of Interleukin-10-afforded protection during dextran sulfate sodium-induced colitis. Frontiers in Immunology9(MAR):, 2018. [Journal: Article] [CI: 33] [IF: 4,7]
DOI: 10.3389/fimmu.2018.00400 SCOPUS: 85042686207


