Differentiation & Cancer
ABOUT
Our aim is to understand the impact of regulatory mechanisms, transcriptional and post-transcriptional, in cancer initiation and progression. We focus mainly on the mechanisms that drive differentiation switches, which constitute the starting point of a significant number of carcinogenic processes, namely in the GI tract. We adopt an integrative approach using clinical specimens, cellular systems and animal models to identify key molecular events and biomarkers that may contribute to understand the biology of cancer and may be translated to patient care.
RESEARCH
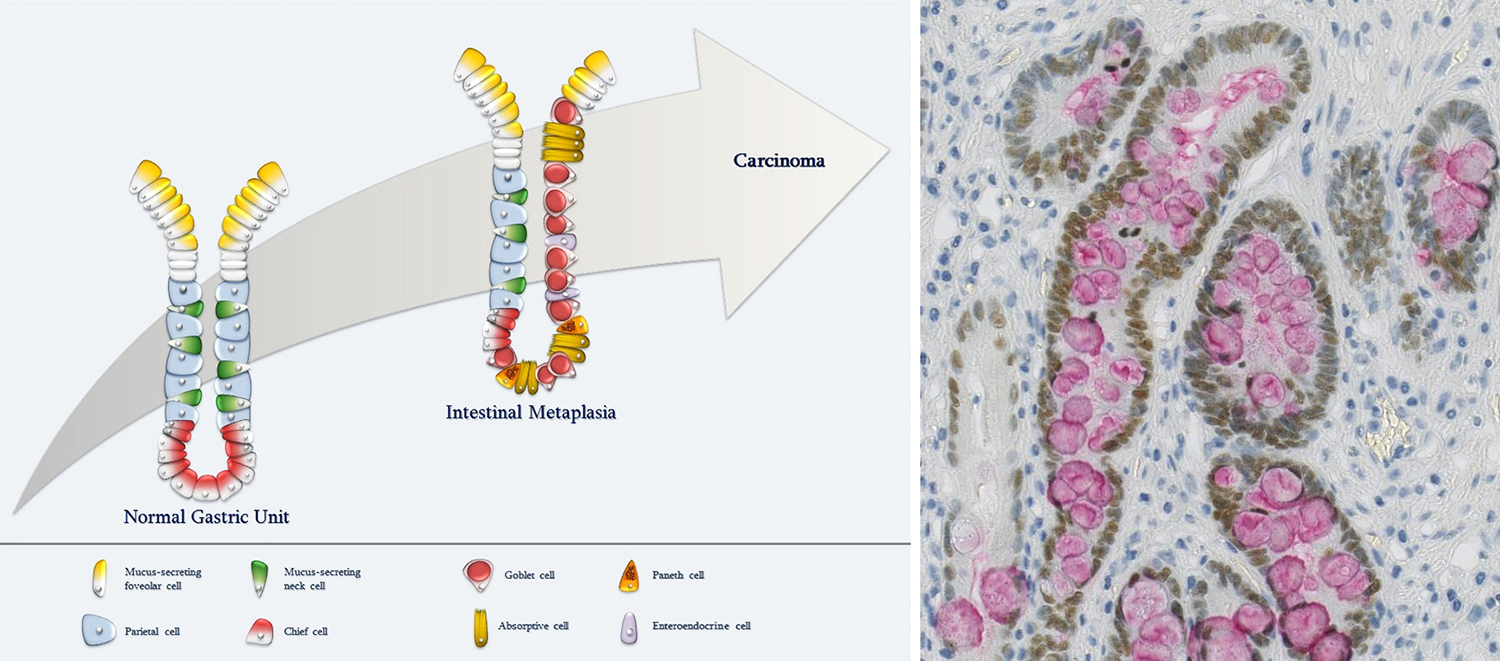
We have identified CDX2 as a prognostic marker in gastric cancer and in colon cancer where it also predicts therapy response. We have also identified SOX2 as an additional biomarker in the context of CDX2 loss of expression, contributing to further stratify patients into groups with different therapy responses, which has important implications in clinical decision-making in the context of colorectal cancer clinical practice. We generated a CDX2 knock-out cellular model, using a genome-editing strategy, that was characterized in depth regarding CDX2 expression modulation and transcriptomic alterations. With this strategy we identified new CDX2 targets and currently we are studying their potential relevance as cancer biomarkers.
We have identified a post-transcriptional regulatory system involved in intestinal stem cell identity and cancer.
We characterized MUC16 glycoforms and new monoclonal antibodies were produced by the group. Studies in ovarian carcinomas extended our knowledge on mucin glycoforms that are relevant and can improve current biomarkers in clinical use. We have been studying the interaction of MUC16 with mesothelin, using the cellular assay “mesothelial clearance”, to understand the role of these proteins in peritoneal metastization of ovarian cancer.
Team
Selected Publications
Prognostic, predictive, and pharmacogenomic assessments of CDX2 refine stratification of colorectal cancer. Molecular Oncology12(9):1639-1655, 2018. [Journal: Article] [CI: 42] [IF: 6]
DOI: 10.1002/1878-0261.12347 SCOPUS: 85052656489
Pinto R., Hansen L., Hintze J., Almeida R., Larsen S., Coskun M., Davidsen J., Mitchelmore C., David L., Troelsen J.T., Bennett E.P.
Precise integration of inducible transcriptional elements (PrIITE) enables absolute control of gene expression. Nucleic Acids Research45(13):, 2017. [Journal: Article] [CI: 21] [IF: 11,6]
DOI: 10.1093/nar/gkx371 SCOPUS: 85026430499
Pereira B., Billaud M., Almeida R.
RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends in Cancer3(7):506-528, 2017. [Journal: Review] [CI: 592] [IF: 7]
DOI: 10.1016/j.trecan.2017.05.003 SCOPUS: 85020932454
Sadio A., Amaral A.L., Nunes R., Ricardo S., Sarmento B., Almeida R., Tsukamoto H., das Neves J.
A mouse intra-intestinal infusion model and its application to the study of nanoparticle distribution. Frontiers in Physiology7(NOV):, 2016. [Journal: Article] [CI: 9] [IF: 4,1]
DOI: 10.3389/fphys.2016.00579 SCOPUS: 85006371437
Camilo V., Garrido M., Valente P., Ricardo S., Amaral A.L., Barros R., Chaves P., Carneiro F., David L., Almeida R.
Differentiation reprogramming in gastric intestinal metaplasia and dysplasia: Role of SOX2 and CDX2. Histopathology66(3):343-350, 2015. [Journal: Article] [CI: 40] [IF: 3,4]
DOI: 10.1111/his.12544 SCOPUS: 84921489545
Ricardo S., Marcos-Silva L., Pereira D., Pinto R., Almeida R., Söderberg O., Mandel U., Clausen H., Felix A., Lunet N., David L.
Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Molecular Oncology9(2):503-512, 2015. [Journal: Article] [CI: 56] [IF: 5,4]
DOI: 10.1016/j.molonc.2014.10.005 SCOPUS: 84921309842
Camilo V., Barros R., Celestino R., Castro P., Vieira J., Teixeira M.R., Carneiro F., Pinto-de-Sousa J., David L., Almeida R.
Immunohistochemical molecular phenotypes of gastric cancer based on SOX2 and CDX2 predict patient outcome. BMC Cancer14(1):, 2014. [Journal: Article] [CI: 37] [IF: 3,4]
DOI: 10.1186/1471-2407-14-753 SCOPUS: 84935505045
Pereira B., Sousa S., Barros R., Carreto L., Oliveira P., Oliveira C., Chartier N.T., Plateroti M., Rouault J.P., Freund J.N., Billaud M., Almeida R.
CDX2 regulation by the RNA-binding protein MEX3A: Impact on intestinal differentiation and stemness. Nucleic Acids Research41(7):3986-3999, 2013. [Journal: Article] [CI: 77] [IF: 8,8]
DOI: 10.1093/nar/gkt087 SCOPUS: 84876542486
Barros R., Freund J.N., David L., Almeida R.
Gastric intestinal metaplasia revisited: Function and regulation of CDX2. Trends in Molecular Medicine18(9):555-563, 2012. [Journal: Review] [CI: 75] [IF: 9,6]
DOI: 10.1016/j.molmed.2012.07.006 SCOPUS: 84865606611
Camilo V., Barros R., Sousa S., Magalhães A.M., Lopes T., Santos A.M., Pereira T., Figueiredo C., David L., Almeida R.
Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis33(10):1985-1992, 2012. [Journal: Article] [CI: 61] [IF: 5,6]
DOI: 10.1093/carcin/bgs233 SCOPUS: 84865579254
Barros R., Da Costa L.T., Pinto-de-Sousa J., Duluc I., Freund J.N., David L., Almeida R.
CDX2 autoregulation in human intestinal metaplasia of the stomach: Impact on the stability of the phenotype. Gut60(3):290-298, 2011. [Journal: Article] [CI: 53] [IF: 10,1]
DOI: 10.1136/gut.2010.222323 SCOPUS: 79851514659


