BioEngineered Surfaces
ABOUT
The BioEngineered Surfaces (BeSurf) group explores nanotechnology-based strategies as alternatives to conventional antibiotics for the treatment of gastric, skin, bone and biomaterial-associated infections.
The group focuses on surface bioconjugation of biomolecules using self-assembled monolayers (SAMs) and biomedical polymers/ceramics to develop antimicrobial coatings or nanoparticles targeting bacterial infection.
Besides fundamental research, the group is also committed to transfer the knowledge created through patent applications in collaboration with industrial partners.
RESEARCH
For the development of these biomaterial surfaces, different approaches are being followed depending on the final medical application:
Biomaterials to fight gastric infection
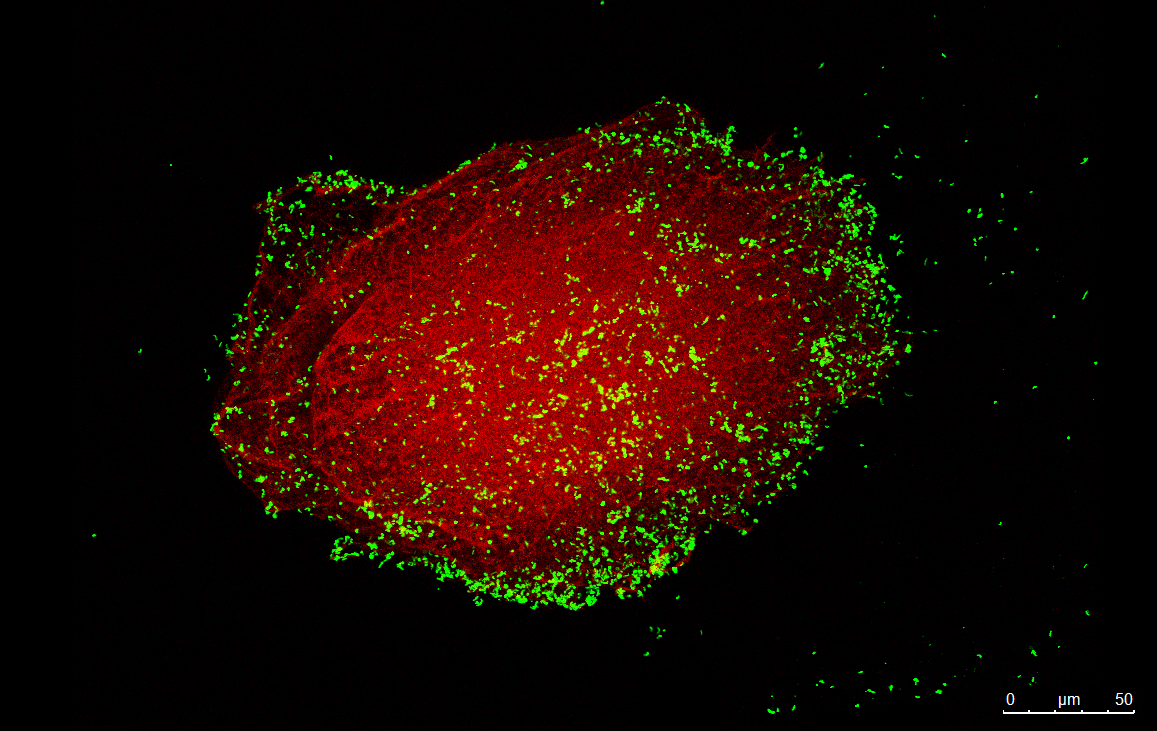
Helicobacter pylori is a Gram-negative bacterium that colonizes the stomach of half of the world population and is responsible for 90% of the gastric cancer burden. The group has been developing antibiotic-free engineered biomaterials (micro/nanoparticles) for H. pylori gastric infection management. Most of the strategies, being specific to H. pylori, can kill them in situ without affecting other bacteria from the gut microbiota. The efficacy of the different approaches is studied in vitro using several H. pylori strains (including clinical isolates) and gastric cell lines and in vivo using a H. pylori-infected mouse model.
Biomaterials to fight wound skin infection
Chronic wounds represent a major healthcare problem affecting 1-2% of the population in developed countries. Infection by antibiotic-resistant bacteria contributes greatly to wound chronicity and is responsible for 33.000 estimated deaths per year in the European Union. Antimicrobial peptides (AMP) are a promising alternative to conventional antibiotics, as they act through non-specific mechanisms and cause virtually no resistance. The group explores AMP grafting on polymeric films, electrospun nanofibers, and nanoparticles as a strategy to reduce AMP aggregation and degradation, while improving local AMP concentration and effectiveness.
Biomaterials to fight bone infection
The group uses innovative bacteriophage-loaded biomaterials to combat bacterial bone infections (osteomyelitis). Phage therapy represents a safe and promising alternative strategy for treating infectious biofilms and multidrug-resistant pathogens, as phages can self-replicate at infection sites, kill target bacteria, and encode biofilm matrix-degrading enzymes. Loading phages into biomaterials, not only shields them from harmful environmental conditions (such as acidic pH, enzymatic degradation, and short serum half-life), but also enables a localized and sustainable release, thereby prolonging their therapeutic activity.
Biomaterial coatings to prevent medical devices associated infection
Biomaterial-associated infection is a major threat in all medical devices since, after surface colonization, pathogens can produce biofilm, protecting them from the host immune system and available therapies. The group has been developing antimicrobial coatings for medical devices to kill bacteria by contact, based on AMPs surface grafting. Different chemical approaches for AMP bioconjugation have been explored to maximize their bactericidal activity after binding.
Team
Selected Publications
Cholesterol Functionalized Nanoparticles Are Effective against Helicobacter pylori, the Gastric Bug: A Proof-of-Concept Study. Advanced Healthcare Materials14(10):, 2025. [Journal: Article] [CI: 1] [IF: 9.6 (*)]
DOI: 10.1002/adhm.202404065 SCOPUS: 85217560687
Fonseca D.R., Alves P.M., Neto E., Custódio B., Guimarães S., Moura D., Annis F., Martins M., Gomes A., Teixeira C., Gomes P., Pereira R.F., Freitas P., Parreira P., Martins M.C.L.
One-Pot Microfluidics to Engineer Chitosan Nanoparticles Conjugated with Antimicrobial Peptides Using “Photoclick” Chemistry: Validation Using the Gastric Bacterium Helicobacter pylori. ACS Applied Materials and Interfaces16(12):14533-14547, 2024. [Journal: Article] [CI: 7] [IF: 8.2]
DOI: 10.1021/acsami.3c18772 SCOPUS: 85187652527
Seabra C.L., Pinho A.S., Nunes C., Amorim I., Pedro N., Henriques P., Monteiro C., Gomes J., Machado C., Gartner F., Pereira L., Reis S., Reis C.A., Touati E., Gonçalves I.C., Parreira P., Martins M.C.L.
Paving the way for a non-antibiotic and microbiota friendly therapy for Helicobacter pylori: In vitro and in vivo performance of lipid nanoparticles. Helicobacter29(1):, 2024. [Journal: Article] [CI: 1] [IF: 4.3]
DOI: 10.1111/hel.13050 SCOPUS: 85182844513
Fonseca D.R., Chitas R., Parreira P., Martins M.C.L.
How to manage Helicobacter pylori infection beyond antibiotics: The bioengineering quest. Applied Materials Today37:, 2024. [Journal: Review] [CI: 10] [IF: 8,3 (*)]
DOI: 10.1016/j.apmt.2024.102123 SCOPUS: 85185511905
Fonseca D.R., Moura A., Leiro V., Silva-Carvalho R., Estevinho B.N., Seabra C.L., Henriques P.C., Lucena M., Teixeira C., Gomes P., Parreira P., Martins M.C.L.
Grafting MSI-78A onto chitosan microspheres enhances its antimicrobial activity. Acta Biomaterialia137:186-198, 2022. [Journal: Article] [CI: 24] [IF: 9,7]
DOI: 10.1016/j.actbio.2021.09.063 SCOPUS: 85118721492
Redondo-Gómez C., Parreira P., Martins M.C.L., Azevedo H.S.
Peptide-based self-assembled monolayers (SAMs): what peptides can do for SAMs and vice versa. Chemical Society Reviews53(8):3714-3773, 2024. [Journal: Review] [CI: 31] [IF: 39]
DOI: 10.1039/d3cs00921a SCOPUS: 85187679238
Costa B., Coelho J., Silva V., Shahrour H., Costa N.A., Ribeiro A.R., Santos S.G., Costa F., Martínez-de-Tejada G., Monteiro C., Martins M.C.L.
Dhvar5- and MSI78-coated titanium are bactericidal against methicillin-resistant Staphylococcus aureus, immunomodulatory and osteogenic. Acta Biomaterialia191:98-112, 2025. [Journal: Article] [CI: 9] [IF: 9.6 (*)]
DOI: 10.1016/j.actbio.2024.11.016 SCOPUS: 85209664352
Zegre M., Barros J., David A.B., Fialho L., Ferraz M.P., Monteiro F.J., Caetano L.A., Gonçalves L., Bettencourt A.
Dual-Loaded Chitosan-Based Nanoparticles: A Novel approach for treating polymicrobial osteomyelitis. International Journal of Pharmaceutics674:, 2025. [Journal: Article] [CI: 4] [IF: 5.2 (*)]
DOI: 10.1016/j.ijpharm.2025.125480 SCOPUS: 105000478621
Alves P.M., Barrias C.C., Gomes P., Martins M.C.L.
How can biomaterial-conjugated antimicrobial peptides fight bacteria and be protected from degradation?. Acta Biomaterialia181:98-116, 2024. [Journal: Review] [CI: 11] [IF: 9.6]
DOI: 10.1016/j.actbio.2024.04.043 SCOPUS: 85192685915
Barros J.A.R., Melo L.D.R.d., Silva R.A.R.d., Ferraz M.P., Azeredo J.C.V.d.R., Pinheiro V.M.d.C., Colaço B.J.A., Fernandes M.H.R., Gomes P.d.S., Monteiro F.J.
Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine: Nanotechnology, Biology, and Medicine24:, 2020. [Journal: Article] [CI: 83] [IF: 6,5]
DOI: 10.1016/j.nano.2019.102145 SCOPUS: 85077500728
Flores M., Ribeiro T.P., Madureira S., Skwira-Rucińska A., Pinto M.T., Monteiro F.J., Laranjeira M.S.
Development of a magnetic biomimetic scaffold for improved bone regeneration via static magnetic stimulation. Ceramics International51(6):7519-7526, 2025. [Journal: Article] [IF: 5.6 (*)]
DOI: 10.1016/j.ceramint.2024.12.188 SCOPUS: 85212204705
Ramôa A.M., Campos F., Moreira L., Teixeira C., Leiro V., Gomes P., das Neves J., Martins M.C.L., Monteiro C.
Antimicrobial peptide-grafted PLGA-PEG nanoparticles to fight bacterial wound infections. Biomaterials Science11(2):499-508, 2022. [Journal: Article] [CI: 21] [IF: 6,6]
DOI: 10.1039/d2bm01127a SCOPUS: 85144032387
Ribeiro T.P., Gomes F.L., Vilarinho R., Salgado C., Martins M.C.L., Moreira J.A., Monteiro F.J., Laranjeira M.S.
Thermoresponsive nanoparticles for targeted and controlled delivery of doxorubicin in triple negative breast cancer: a 2D and 3D in vitro evaluation. Drug Delivery and Translational Research16(1):108-123, 2026. [Journal: Article] [IF: 5.5 (*)]
DOI: 10.1007/s13346-025-01930-9 SCOPUS: 105012174111


