BioEngineered Surfaces
ABOUT
The BioEngineered Surfaces (BeSurf) group focuses on nanotechnology approaches to fight infection and improve hemocompatibility of medical devices.
The group is expert in surface bioconjugation of biomolecules using self-assembled monolayers (SAMs) and biomedical polymers to create surfaces that will guide specific protein/cell binding and control bacterial adhesion/biofilm and/or thrombus formation.
Besides fundamental research, the group is also committed to transferring the knowledge created through patent applications in collaboration with industrial partners.
For the development of these biomaterial surfaces, different approaches are being followed depending on the final medical application:
RESEARCH
Biomaterials to fight gastric infection
Helicobacter pylori is a Gram-negative bacterium that colonizes the stomach of half of the world population and is responsible for 90% of the gastric cancer burden.
The group has been developing antibiotic-free engineered biomaterials (micro and nanoparticles) for H. pylori gastric infection management. These strategies, being specific to H. pylori, are able to kill them in situ or to capture and remove them from infected hosts, without affecting other bacteria from the gut microbiota. The efficacy of the different approaches is studied in vitro using several H. pylori strains (including clinical isolates) and gastric cell lines and in vivo using an H. pylori-infected mice model.
Biomaterials to fight wound skin infection
Chronic wounds represent a major healthcare problem affecting 1-2% of the population in developed countries. Infection by antibiotic-resistant bacteria contributes greatly to wound chronicity and is responsible for 33.000 estimated deaths per year in the European Union. Antimicrobial peptides (AMP) are a promising alternative to conventional antibiotics, as they act through non-specific mechanisms and cause virtually no resistance. The group explores AMP grafting on polymeric films and nanoparticles as a strategy to reduce AMP aggregation and degradation, while improving local AMP concentration and effectiveness.
Biomaterial coatings to prevent medical devices-associated infections and thrombus formation
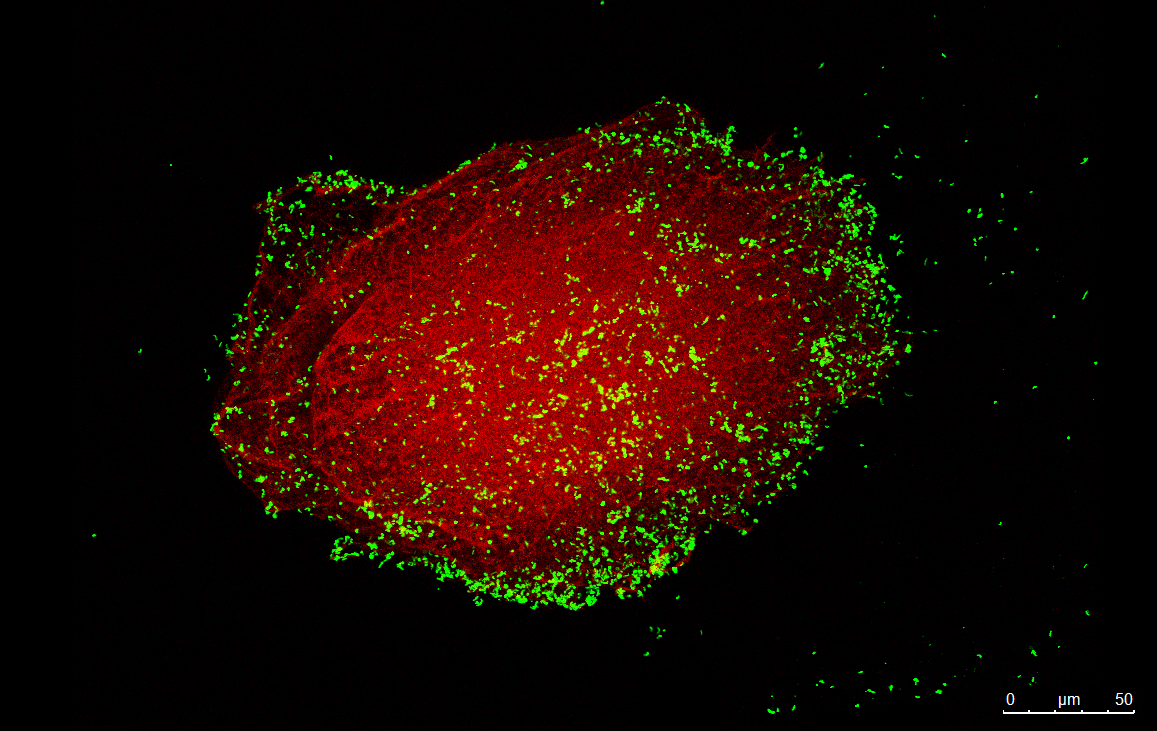
Biomaterials-associated infection is a major threat in all medical devices since, after surface colonization, pathogens can produce biofilm protecting them from the host immune system and available therapies. Moreover, thrombus formation is also a serious concern associated with blood-contacting medical devices. The group has been developing biomaterial coatings with i) antimicrobial properties to kill bacteria by contact or ii) anti-adhesive features to avoid bacterial adhesion and thrombus formation.
Antimicrobial coatings have been developed based on AMPs grafting onto biomaterials, namely for orthopedic applications. Different chemical approaches for AMP bioconjugation have been explored to maximize their bactericidal activity after binding.
Anti-adhesive coatings are investigated using two main approaches namely i) albumin-binding coatings due to albumin “passivant” effect and ii) polysaccharides coatings based on extracellular polymer released by marine cyanobacteria.
ACTIVE PROJECTS:
- Bio2Skin Advanced - Development of a new generation of adhesives with antiseptic, regenerative and adhesive proprieties to prevent and/or treat skin lesions caused by the use of conventional medical adhesives. Project in co-promotion with the company BestHealth4u (NORTE-01-0247-FEDER-047225)
- NanoPyl® - A breakthrough approach for Helicobacter pylori control. (CaixaResearch Validate 2022, La Caixa Foundation)
- BIOGEN - Biofilm genetic regulation in the treatment of Chronic Wound Infection (FCT, 2022.04739.PTDC)
Team
Selected Publications
One-Pot Microfluidics to Engineer Chitosan Nanoparticles Conjugated with Antimicrobial Peptides Using “Photoclick” Chemistry: Validation Using the Gastric Bacterium Helicobacter pylori. ACS Applied Materials and Interfaces16(12):14533-14547, 2024. [Journal: Article] [CI: 4] [IF: 8.3 (*)]
DOI: 10.1021/acsami.3c18772 SCOPUS: 85187652527
Redondo-Gómez C., Parreira P., Martins M.C.L., Azevedo H.S.
Peptide-based self-assembled monolayers (SAMs): what peptides can do for SAMs and vice versa. Chemical Society Reviews53(8):3714-3773, 2024. [Journal: Review] [CI: 5] [IF: 40.4 (*)]
DOI: 10.1039/d3cs00921a SCOPUS: 85187679238
Alves P.M., Fonseca D.R., Bidarra S.J., Gomes A., Gomes P., Barrias C.C., Martins M.C.L.
Norbornene-chitosan nanoparticles with and without a conjugated VEGF-peptide analog to promote vascularization. Materials Today Chemistry36:, 2024. [Journal: Article] [CI: 2] [IF: 7,3 (*)]
DOI: 10.1016/j.mtchem.2024.101942 SCOPUS: 85184149781
Alves P.M., Barrias C.C., Gomes P., Martins M.C.L.
How can biomaterial-conjugated antimicrobial peptides fight bacteria and be protected from degradation?. Acta Biomaterialia181:98-116, 2024. [Journal: Review] [CI: 2] [IF: 9.4 (*)]
DOI: 10.1016/j.actbio.2024.04.043 SCOPUS: 85192685915
Seabra C.L., Pinho A.S., Nunes C., Amorim I., Pedro N., Henriques P., Monteiro C., Gomes J., Machado C., Gartner F., Pereira L., Reis S., Reis C.A., Touati E., Gonçalves I.C., Parreira P., Martins M.C.L.
Paving the way for a non-antibiotic and microbiota friendly therapy for Helicobacter pylori: In vitro and in vivo performance of lipid nanoparticles. Helicobacter29(1):, 2024. [Journal: Article] [CI: 1] [IF: 4.3 (*)]
DOI: 10.1111/hel.13050 SCOPUS: 85182844513
Fonseca D.R., Chitas R., Parreira P., Martins M.C.L.
How to manage Helicobacter pylori infection beyond antibiotics: The bioengineering quest. Applied Materials Today37:, 2024. [Journal: Review] [CI: 3] [IF: 8,3 (*)]
DOI: 10.1016/j.apmt.2024.102123 SCOPUS: 85185511905
Barbosa M., Alves P.M., Costa F., Monteiro C., Parreira P., Teixeira C., Gomes P., Martins M.C.L.
Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings. Pharmaceutics15(5):, 2023. [Journal: Article] [CI: 4] [IF: 5,4 (*)]
DOI: 10.3390/pharmaceutics15051510 SCOPUS: 85160394267
Pinho A.S., Seabra C.L., Nunes C., Reis S., L. Martins M.C., Parreira P.
Helicobacter pylori biofilms are disrupted by nanostructured lipid carriers: A path to eradication?. Journal of Controlled Release348:489-498, 2022. [Journal: Article] [CI: 10] [IF: 10,8]
DOI: 10.1016/j.jconrel.2022.05.050 SCOPUS: 85132423536
Fonseca D.R., Moura A., Leiro V., Silva-Carvalho R., Estevinho B.N., Seabra C.L., Henriques P.C., Lucena M., Teixeira C., Gomes P., Parreira P., Martins M.C.L.
Grafting MSI-78A onto chitosan microspheres enhances its antimicrobial activity. Acta Biomaterialia137:186-198, 2022. [Journal: Article] [CI: 17] [IF: 9,7]
DOI: 10.1016/j.actbio.2021.09.063 SCOPUS: 85118721492
Ramôa A.M., Campos F., Moreira L., Teixeira C., Leiro V., Gomes P., das Neves J., Martins M.C.L., Monteiro C.
Antimicrobial peptide-grafted PLGA-PEG nanoparticles to fight bacterial wound infections. Biomaterials Science11(2):499-508, 2022. [Journal: Article] [CI: 7] [IF: 6,6]
DOI: 10.1039/d2bm01127a SCOPUS: 85144032387
Chitas R., Nunes C., Reis S., Parreira P., Martins M.C.L.
How Charge, Size and Protein Corona Modulate the Specific Activity of Nanostructured Lipid Carriers (NLC) against Helicobacter pylori. Pharmaceutics14(12):, 2022. [Journal: Article] [CI: 4] [IF: 5,4]
DOI: 10.3390/pharmaceutics14122745 SCOPUS: 85144862441
Alves P.M., Pereira R.F., Costa B., Tassi N., Teixeira C., Leiro V., Monteiro C., Gomes P., Costa F., Martins M.C.L.
Thiol-Norbornene Photoclick Chemistry for Grafting Antimicrobial Peptides onto Chitosan to Create Antibacterial Biomaterials. ACS Applied Polymer Materials4(7):5012-5026, 2022. [Journal: Article] [CI: 14] [IF: 5]
DOI: 10.1021/acsapm.2c00563 SCOPUS: 85135691768